Environmental Monitoring of Aseptic Processes
What is environmental monitoring for medical products & medical devices?
Environmental monitoring is the tools and techniques used to observe an environment, characterize an environment’s quality, and ensure that an environment meets established acceptance criteria. Environmental monitoring for medical products and medical devices covers the acceptance criteria for manufacturing throughout the entire lifecycle of the product, from raw materials to end-use or expiration. Environmental monitoring is an important quality control metric and is particularly critical for ensuring your medical device or product is microbe-free during various phases of manufacturing, packaging, transportation, and storage.
What are aseptic processes?
Aseptic processes are methods or procedures that are undertaken in a sterile environment (such as an isolator). The aseptic sterile environment is maintained through specialized equipment that prevents microbial material from technicians, raw materials, or machinery from contaminating medical devices or products.
The terms aseptic and sterile are not synonymous. While both sterile and aseptic products will prevent microbial contamination following use, the processes by which microbial contamination is prevented are different. The term sterile means a complete absence of viable microorganisms or microbes that have the potential to reproduce. Thus, sterile products are often chemically or heat sterilized after being placed in their final packaging. The chemical or heat sterilization kills any microorganisms inside the products (obtained during manufacturing and packaging). This chemical or heat sterilization process after final product packaging is known as terminal sterilization.
However, an aseptic process prevents contamination by the exclusion of microorganisms. Though the definitions for aseptic and sterile are not the same, sterile is used interchangeably with aseptic. Indeed, many products labeled as sterile are manufactured by aseptic processing rather than terminal sterilization.
What are examples of medical products manufactured in aseptic environments?
- Pharmaceutical sterile products
- Bulk sterile drug substances
- Sterile intermediates
- Excipients
- Medical devices
- Biologics
Note that the USP guidelines referenced for microbiological evaluation should be applied only to ISO-classified clean environments, restricted-access barrier systems (RABS), and isolators used for aseptic processing. Other clean environments are not required to meet the levels of contamination control required for aseptically produced sterile products. For products, such as oral tablets, topicals, or nasal sprays, that require non-sterile processing, please see our article on non-sterile processing HERE.
Why is environmental monitoring for aseptic processes important for your medical device or product?
Aseptic processing requires the exclusion of microorganisms from the manufacturing methods. Microorganisms must be prevented from entering open containers or product materials during processing. Thus, product bioburden and the bioburden of the manufacturing environment impact the risk of unacceptable microbial contamination. Since products made from aseptic processing will not undergo terminal sterilization, it is critical to keep aseptic processing environments free of microbes to alleviate the risk of patient infection following product use. In advanced aseptic processing, operators wearing cleanroom garments are not needed or permitted for aseptic processing. For more information on the top sources of microbial contamination in manufacturing environments, visit our article HERE.
What do you need to evaluate for environmental monitoring for aseptic processes?
Microbial contamination is inevitable in environments with human operators. Even the most cautious clean-room environment design and fastidious operation will not eliminate the shedding of microorganisms if human operators are present. As a result, zero contamination at aseptic locations during every aseptic process is unrealistic.
There are no perfect means to verify that an aseptic processing environment and the product-contact surfaces within that environment are sterile all the time. Technically, monitoring results can neither prove nor disprove sterility. However, manufacturers should review environmental monitoring results frequently so that the facility operates in a validated state of control.
Due to monitoring limitations, manufacturers cannot rely on monitoring, statistics, or aseptic processing simulations to assure a sterility level. Sterility assurance is best created by focusing on human-borne contamination and designing the facility to mitigate risk from this contamination.
Microbial contamination risk is greatly mitigated by reducing or eliminating human interventions through proper equipment design and by providing sufficient air exchanges per hour for the personnel population within the facility. Effective movement of personnel and materials, the proper control of temperature and humidity, and periodic sanitization are other contamination risk mitigation factors that can interrupt the chain of infection. Table 3 of USP 1116 (reproduced as Table 1 below) gives the suggested contamination recovery rates in various aseptic environments.
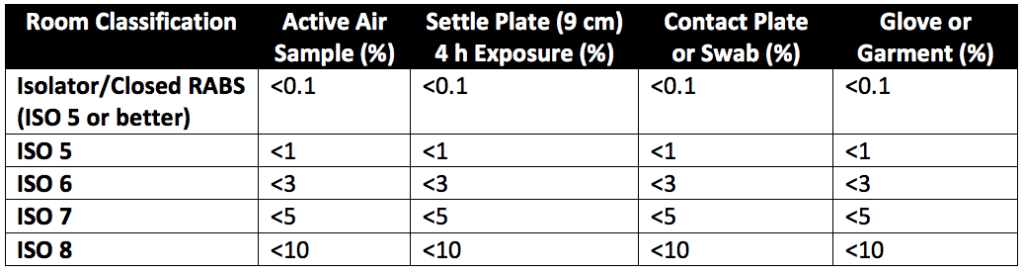
Environmental monitoring of aseptic processes involves:
- Establishing mandatory training for operators on proper cleanroom garb and practices for aseptic processes.
- Creating an evaluation program (or protocol) for microbial contamination (control parameters, sampling plan, and sampling sites).
- Creating protocols for culture conditions and quantification of microbes after sampling.
How are environmental monitoring tests performed?
Regarding microbial sampling of aseptic environments, multiple sampling methods can quantify and control the microbiological status of controlled environments. Almost all sampling methods rely on the recovery and growth of microorganisms. Monitoring microbes in this manner is tricky as the recovered microbes may be in a damaged state and not representative of the actual population of viable microbes in the facility. Additionally, microbe recovery, in general, can be difficult. Because of the variety in microbiological sampling equipment and methods, a set quantitative number cannot be used to qualify or disqualify any aseptic facility. Instead, risk assessments of manufacturing environments are made over time and on a case-by-case basis. The contamination recovery rate metric for each aseptic facility should be established based on a review of actual findings within the facility. Contamination recovery rates track ongoing performance and refine the microbiological control (foster improvements) at the facility. Contamination recovery rate levels typically stabilize within a normal range of variability when optimum operational conditions are implemented.
For environmental monitoring testing, samples of the air and samples of surfaces are taken to assess the types and concentrations of microorganisms in the environment. Regarding air sampling, there are no standard methods for air sampling. The scientific literature suggests that the results from different air sampling methods are highly variable. Thus, manufacturers cannot assume that similar air sample volumes taken by different methods will produce similar rates of microbial recovery. Microbial recovery and survival are affected both by air sampling methods and by air samplers from other suppliers. Indeed, air sampler suppliers design their systems to meet different requirements, as there are no standardized requirements for air sampling. Additionally, sample-to-sample variations in microbial sampling is wide, leading to poor accuracy, precision, sensitivity, and limits of detection for microbial sampling methods used to monitor aseptic processes for healthcare products. Please see our article on airborne microorganism sampling for additional details on seven different air sampling methods.
Like air sampling methods, surface sampling methods are also not standardized. Some techniques use dry swab methods, and some use wet swab methods for contact plates. Further, different media are used with the contact plates. However, multiple contact plate samples should have similar results under identical microbial recovery and growth conditions. In general, surface monitoring has poor microbial recovery rates. Under scientific conditions, less than 50% of microbes are recovered, even when relatively high inoculum levels are used. In actual production environments where organisms are chemically and mechanically stressed, recovery rates may be lower. Thus, surface sampling is essential but may not play as great a role in controlling microbial contamination within aseptic environments as air sampling.
Water sampling may also occur to ensure water used for manufacturing and cleaning is sufficiently microbe-free. For an overview of water sampling vs. air sampling, please visit our comparison article HERE.
Summary
Overall, environmental monitoring for aseptic processes evaluates whether ISO-certified clean environments (such as restricted-access barrier systems (RABS) and isolators used for aseptic processing) are suitable for preventing microbial contamination of medical products during manufacturing, packaging, or testing. For environmental monitoring testing, samples of the air and samples of surfaces are taken to assess the types and concentrations of microorganisms in the environment. There is heavy variance in microorganism retrieval for different sampling methods. As a result, manufacturers cannot assume that they will achieve the same microbial monitoring results using multiple sampling methods or microbial sampling equipment from different suppliers. All in all, make sure you choose a contract testing organization that can support you with appropriate and consistent environmental monitoring for aseptic processes for your medical product or medical device.
Ethide Labs is a contract testing organization that specializes in Environmental Monitoring. Ethide Labs provides in-vitro cytotoxicity tests in-house and outsources in-vivo cytotoxicity work for toxicity testing of medical devices, products, and drugs. Ethide Labs also offers Microbiology Testing, Sterilization Validations, Bioburden Testing, Bacterial Endotoxin Testing, EO Residual Testing, Package Integrity Testing & Cytotoxicity Testing services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
United States Pharmacopeial Convention. <1116> Microbiological Control & Monitoring of Aseptic Processing Environments. Rockville, MD, USA. 2021. (USPC <1116>).
Share this in your social networks


