Bioburden and USP 60
Microbiological Examination of Nonsterile Products-Tests for Burkholderia Cepacia Complex
What does USP 60 Cover?
USP 60 details methods that determine the absence or the presence of Burkholderia cepacia complex (Bcc) in various samples. USP 60 provides methods for identifying Bcc members from multiple pharmaceutical preparations, such as aqueous, oral, cutaneous, and nasal.
Procedures and Tests Covered in USP 60
USP 60 covers testing methods for three strains of Burkholderia. These three strains of Burkholderia are Burkholderia cepacia, Burkholderia cenocepacia, and Burkholderia multivorans. Procedures detailed in USP 60 include test strain preparation, test controls, test methods for growth-promoting properties, testing methods for inhibitory properties, and test methods for indicative properties. Information on recommended culture media and agar preparation is also included in this standard.
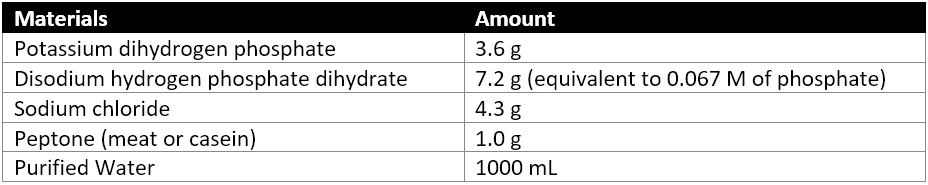
When preparing test strains for Bcc, strains should be within five passages of the original strain cultured. Recommended standards to be purchased for Burkholderia cepacian test strains are ATCC 25416, NCTC 10743, and CIP 80.24. ATCC BAA-245 and LMG 16656 are the standards recommended to be bought for Burkholderia cenocepacia test strains. Recommended standards to be purchased for Burkholderia multivorans test strains are ATCC BAA-247, LMG 13010, CCUG 34080, CIP 105495, and DSM NCTC 13007. Test strains are grown in Soybean-Casein Digest Broth or Soybean-Casein Digest Agar at 30-35°C for 18-14hrs. Phosphate Buffer Solution pH7.2 or Buffered Sodium Chloride-Peptone Solution pH 7.0 are used for test suspensions. Test suspensions will be used within 24 hours of preparation. Negative controls of Phosphate Buffer Solution are used to confirm that there is no microbial growth resulting from the culture environment or unforeseen contamination of the solutions used in sample preparation.
Growth Promotion Testing
The Surface-Spread Method is used for growth promotion testing. You can find additional details about the Surface-Spread Method in USP 61. After agar plates have been prepared as described in USP 61, a small number (not more than 100 colony forming units (CFU)) of the test strain is added to each agar plate. Agar plates are then incubated for the briefest amount of time possible for each microorganism tested. Burkholderia cepacian, Burkholderia cenocepacia, or Burkholderia multivorans are used as test strains for growth-promoting and indicative tests. For inhibitory tests, pseudomonas aeruginosa and staphylococcus aureus are used as test strains.
Inhibitory Properties Testing
Bcc samples are exposed to either Pseudomonas aeruginosa or Staphylococcus aureus at a 100 CFU dilution. Inhibition of growth of the indicated microorganism is observed and assessed via CFU.
Indicative Properties Testing
The surface-spread method is used to add not more than 100 CFU of the microorganism being evaluated to each plate. Plates are then incubated at the temperature and time specified in the test. These colonies are then assessed based on Bcc colonies’ known appearance to determine if Bcc is present.
Product Testing
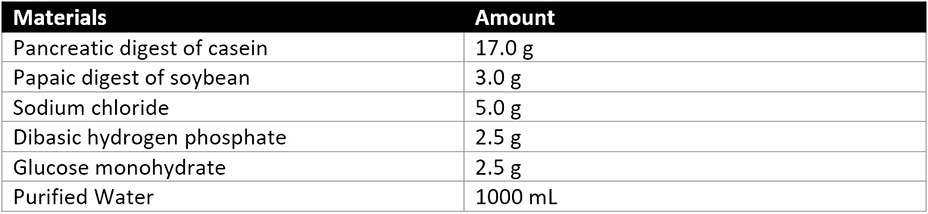
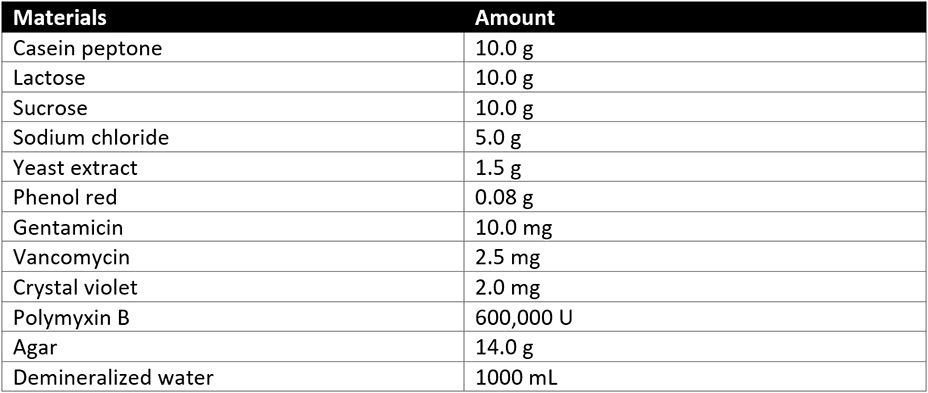
A dilution of 1 in 10 of not less than 1 gram of the product being assessed is prepared. Soybean-casein digest broth is added to the product dilution. The product dilution in the soybean-casein digest broth is then incubated at 30-35°C. After incubations, the soybean-casein digest broth with product dilution is subcultured on Burkholderia Cepacian Selective Agar and incubated at 30-35°C for 2-3 days. USP 60 details that Bcc is indicated by the growth of “greenish-brown colonies with yellow halos or white colonies surrounded by pink-red zones” on the Burkholderia cepacian selective agar. Any Bcc growth is further confirmed by identification tests described above. The product passes the Bcc test if there are no Bcc colonies present or if identification tests (to confirm the presence of Bcc) are Bcc free.
Product Testing In The Presence of Bcc
First, the product sample is prepared as described above under the product testing section. Next, each of the Bcc test strains is added to their recommended growth medium. Product samples are then exposed to Bcc test strains individually using not less than 100 CFU in the test strain preparation. A short incubation period is performed, and Bcc growth is assessed. Any antimicrobial activity of the product following Bcc growth will require a secondary assay where the antimicrobial activity is neutralized and Bcc growth reassessed.
How does USP 60 relate to bioburden testing?
The “bio” in bioburden refers to live biological organisms, and the “burden” in bioburden refers to the concentration of the viable biological organisms. The higher the concentration of viable organisms on a device or product, the higher the burden is to kill those organisms. The USP 60 pharmacopeia methods outline the specifics of how the microorganism testing for the Burkholderia cepacian complex should be carried out. Specifically, USP 60 describes tests for product testing, as well as growth, inhibition, and indication assays for three strains of Bcc. Media and agar preparations for the tests detailed in this guidance are also provided. When performing testing in-house or outsourcing your testing to a contract testing facility, it is important to make sure the USP 60 pharmacopeia methods are met for all bioburden testing. Additionally, bioburden testing should meet pharmacopeia methods from USP 61 and USP 62.
Ethide Labs is a contract testing organization that specializes in Bioburden Testing. Ethide Labs also offers Bacterial Endotoxin Testing, Environmental Monitoring, Sterilization Validations, Microbiology Testing, EO Residual Testing, Package Integrity Testing & Cytotoxicity Testing services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
United States Pharmacopeial Convention. <60> Microbiological Examination of Nonsterile Products- Tests for Burkholderia Cepacia Complex. Rockville, MD, USA. 2021. (USPC <60>)
Share this in your social networks


